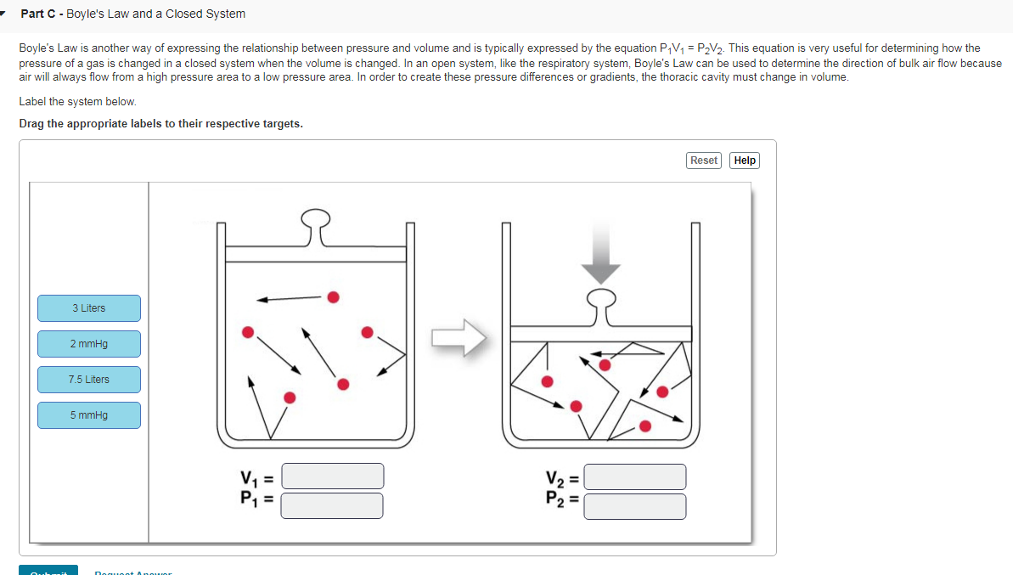
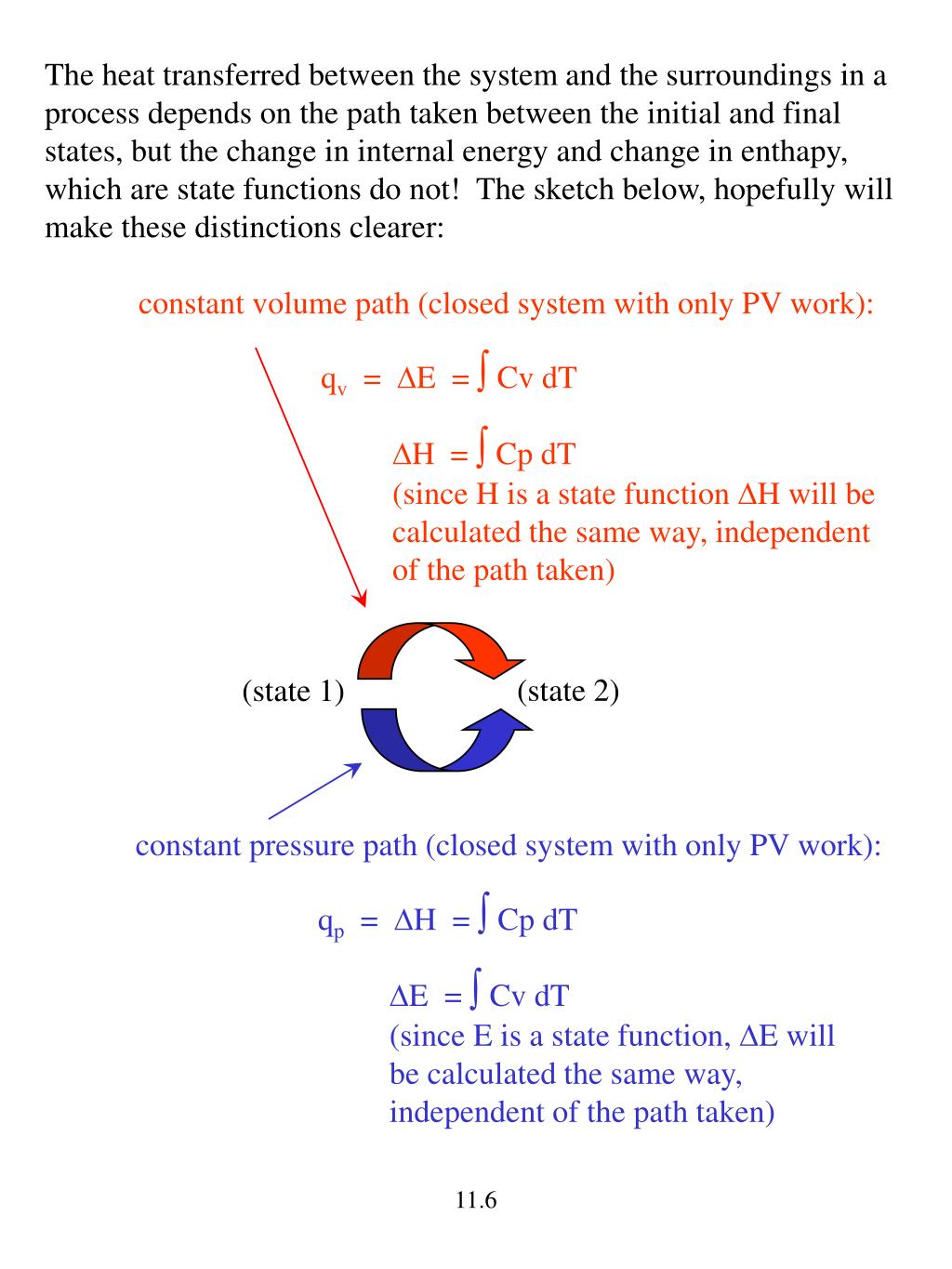
Can The Volume Of A Closed System Change
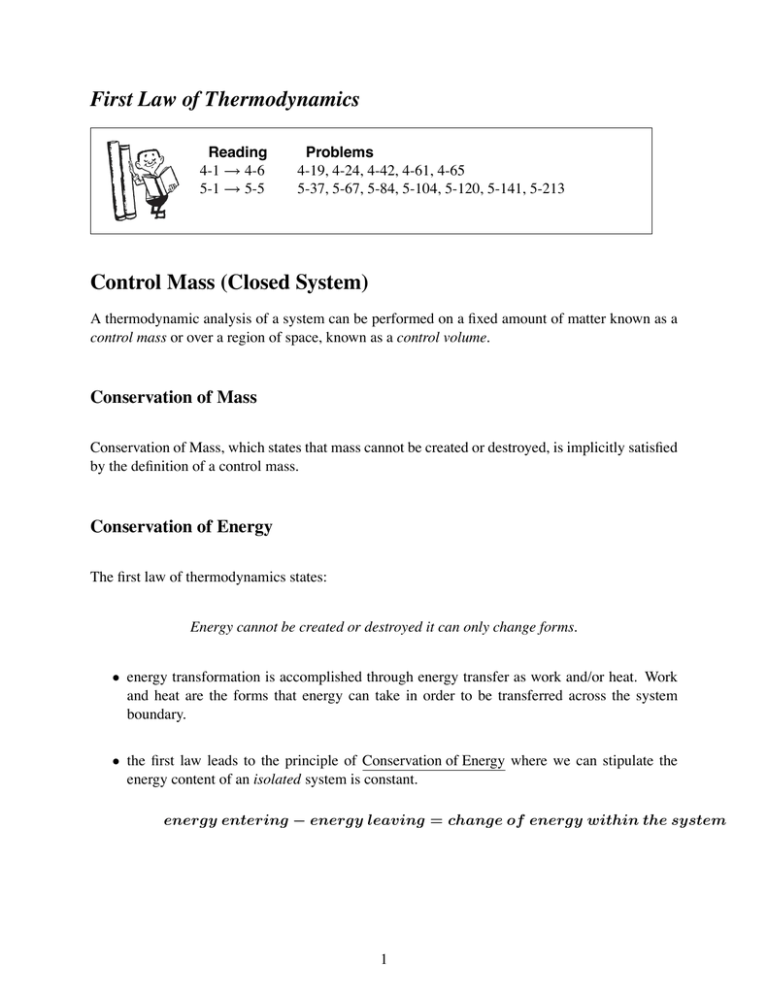
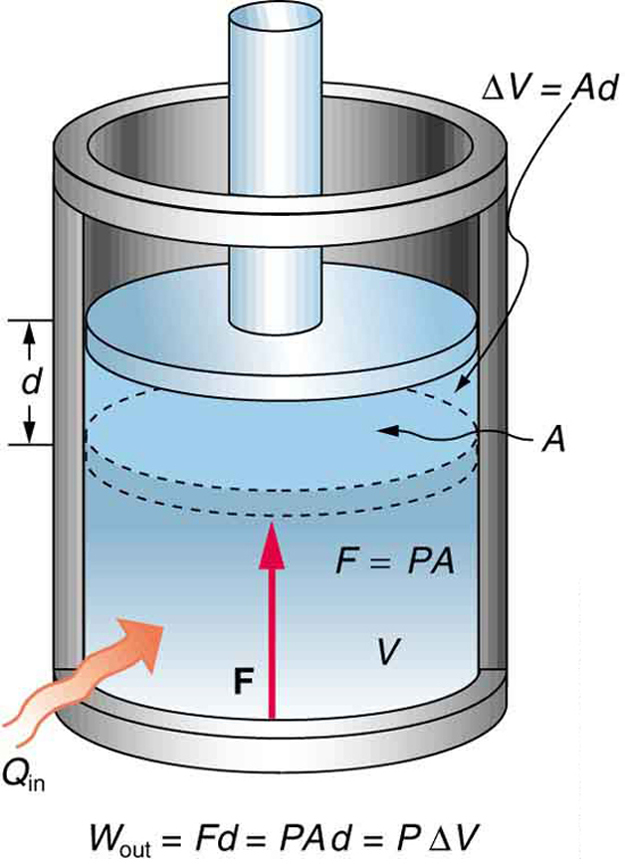
Can the volume of a closed system change. It does not matter whether material flows through the control volume or the control volume passes through the material. Many chemical reactions are not however carried out in sealed containers at constant volume but in open containers at a more or less constant pressure of. An isochoric process also called a constant-volume process an isovolumetric process or an isometric process is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant.
This is how air conditioners work. From the plot we can see that the system naturally tends to roll down a Gibbs energy hill until it reaches the lowest point. For the following methods of system sizing it is necessary to take before and after samples for analysis of the sizing agent.
The change in concentration is used to calculate system volume. When the pressure decreases then the. Dm dt 0 2.
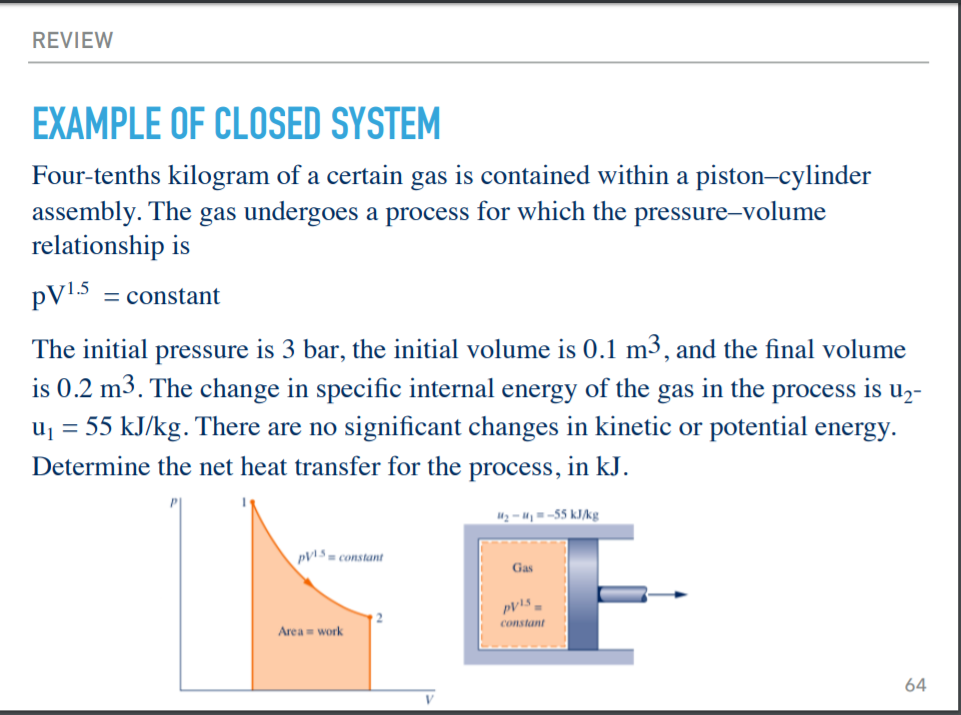
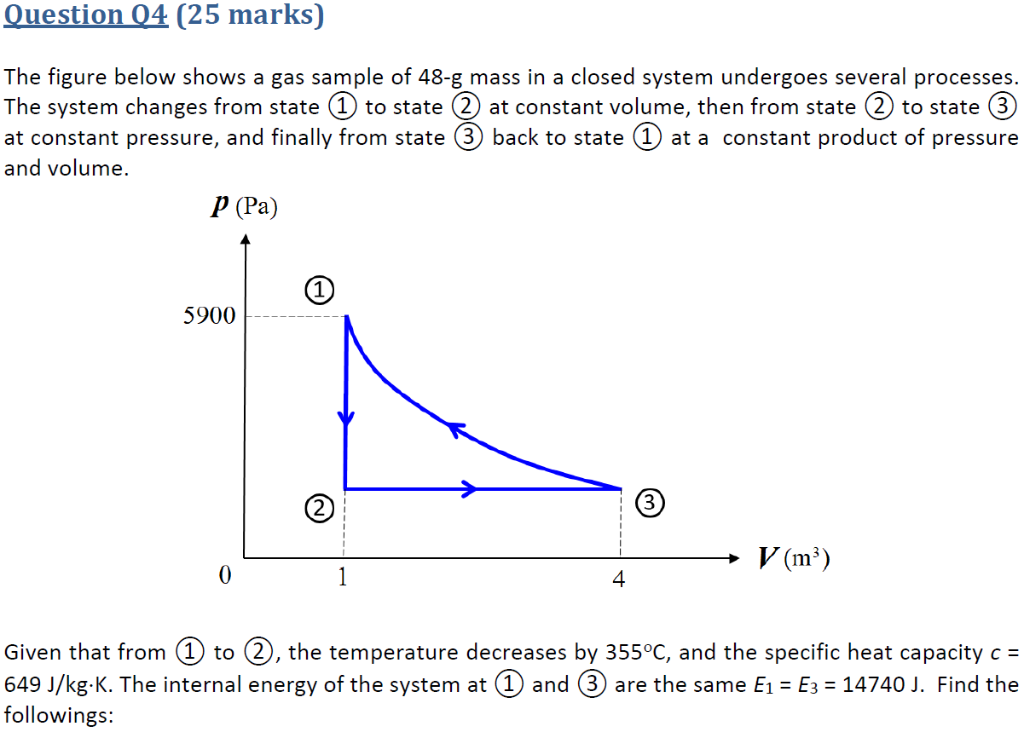
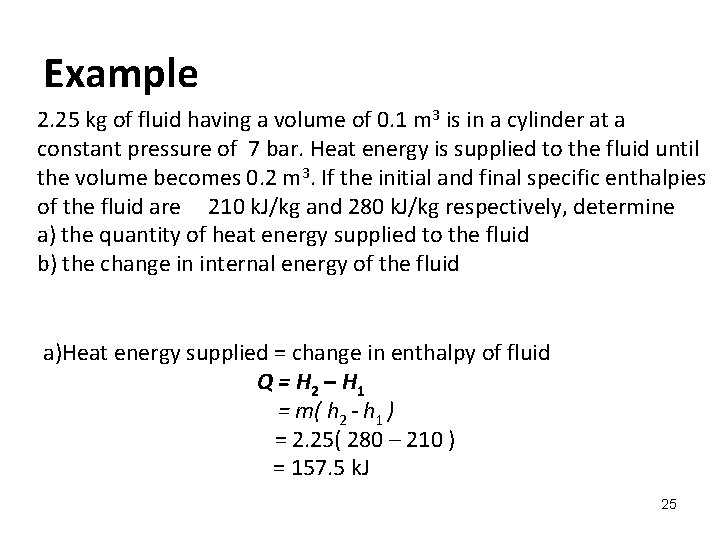
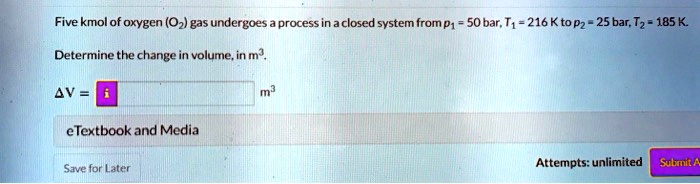
Determine the work for the process in kJ if a n15 b n10 and c n0. It can do anything you want it to do. To sum it up- we can write -.
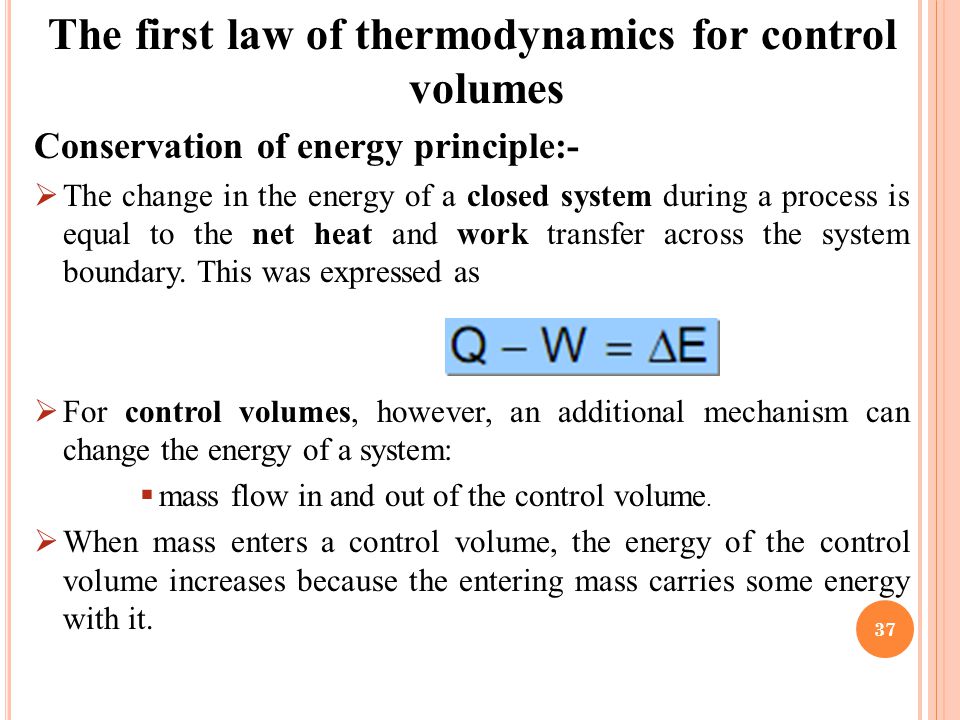
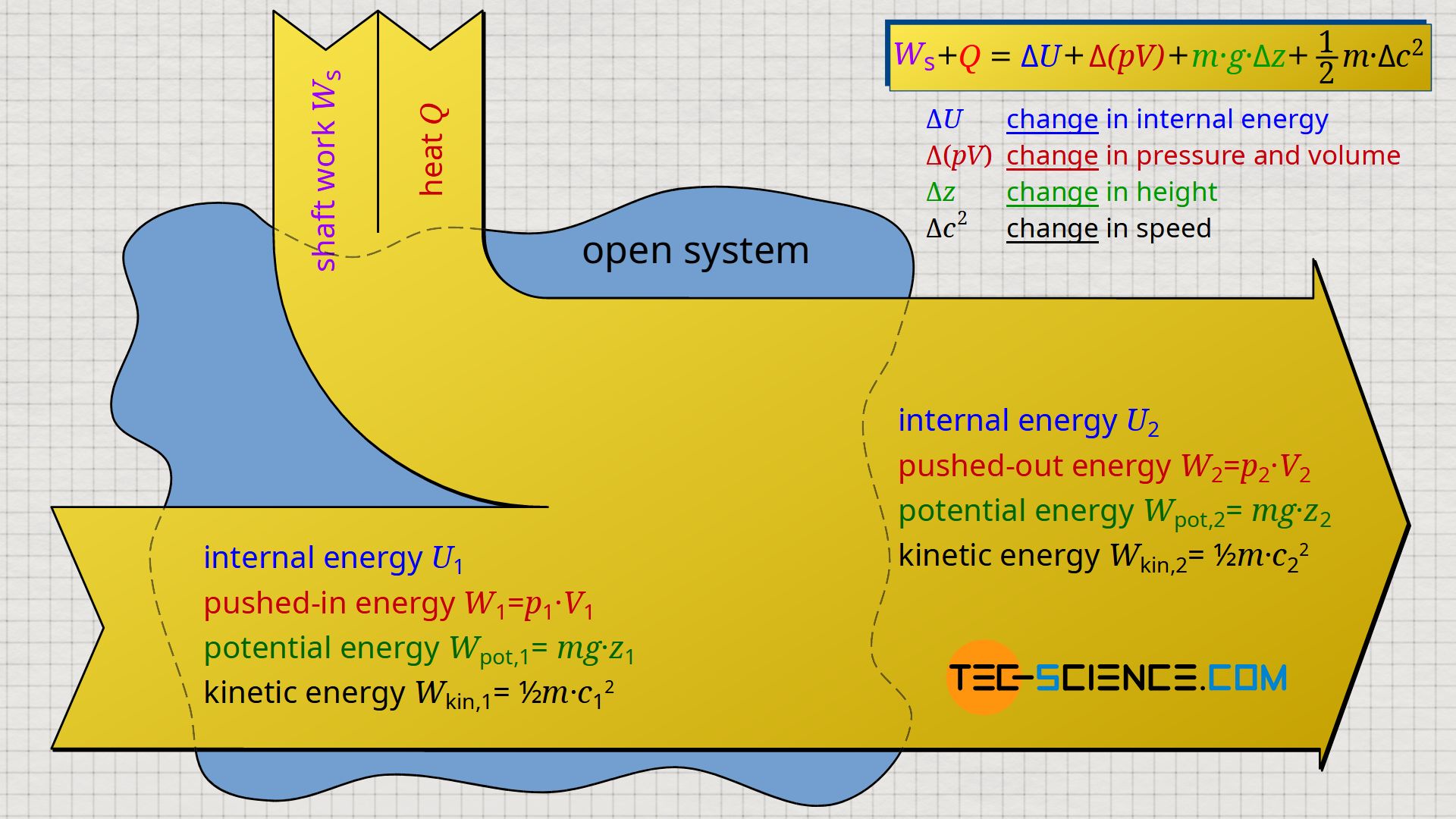
Water Volume in an Closed Loop System JohnGP Mechanical 3 Oct 13 2256 Once you add up all the bits typically I would think that an expansion tank would be included in the loop sized to take any increase in liquid volume. You introduce a control volume so that you can focus on what happens inside it but you must take account of anything that passes through the control volume. There are several ways to determine an accurate system volume for a closed loop.
For example when the pressure increases then the temperature also increases. Heat rejection is the only way that the entropy of a fixed mass can be decreased. Begingroup John Rennie then can I say If a thermodynamic system consists of charges and has an electricmagnetic field then it is not necessary for the volume to change.
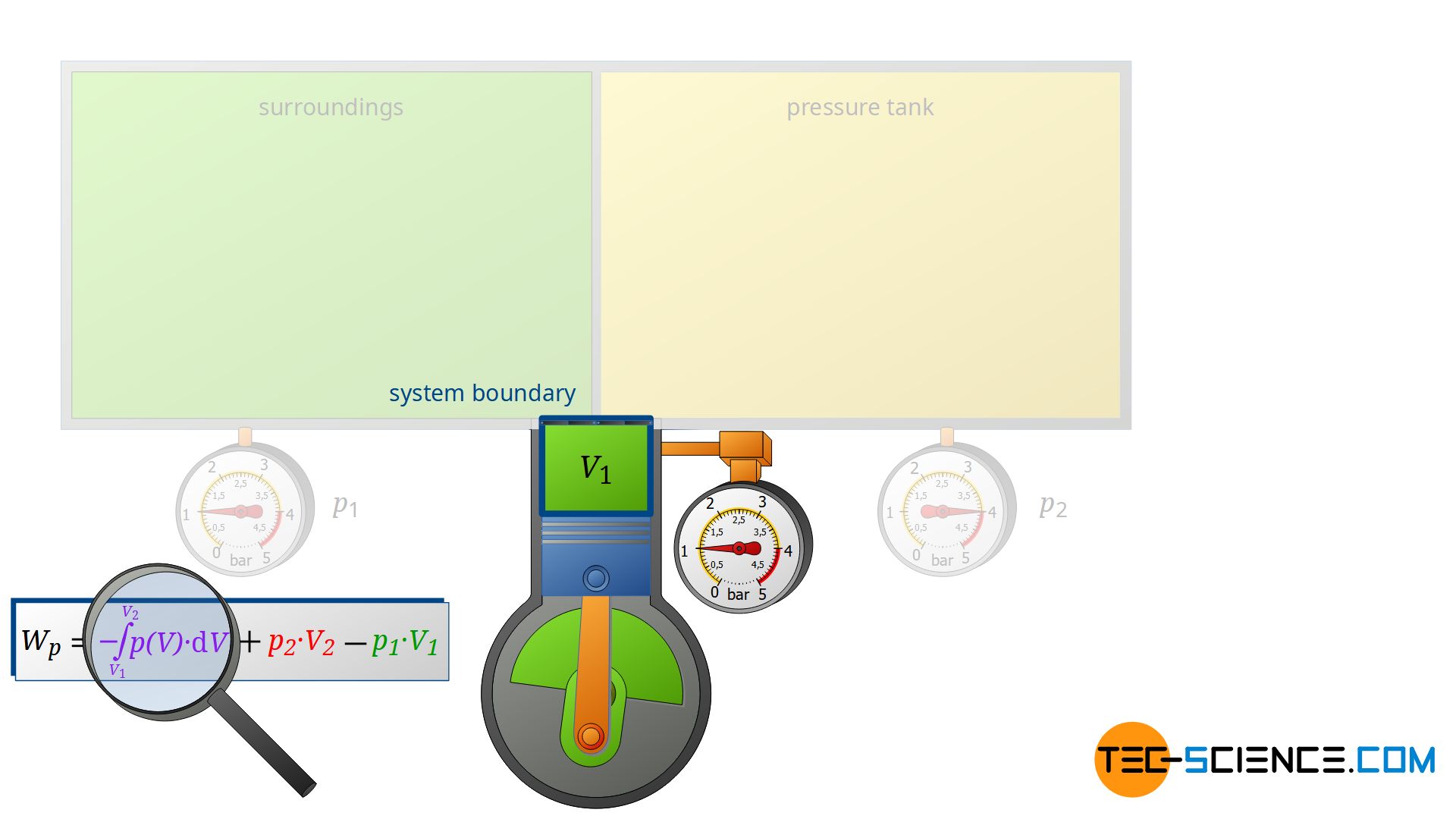
Assume that i the gas is a closed system ii the moving boundary is only work mode and iii the expansion is polytropic. This rela-tion is valid whether the control volume is fixed moving or deforming.
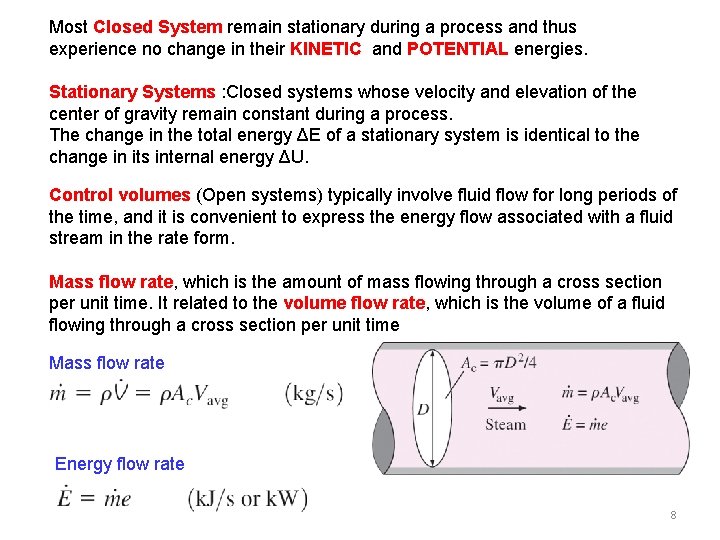
As the temperature or pressure of the system changes the volume of the system will change and with it the shape of the system boundary.
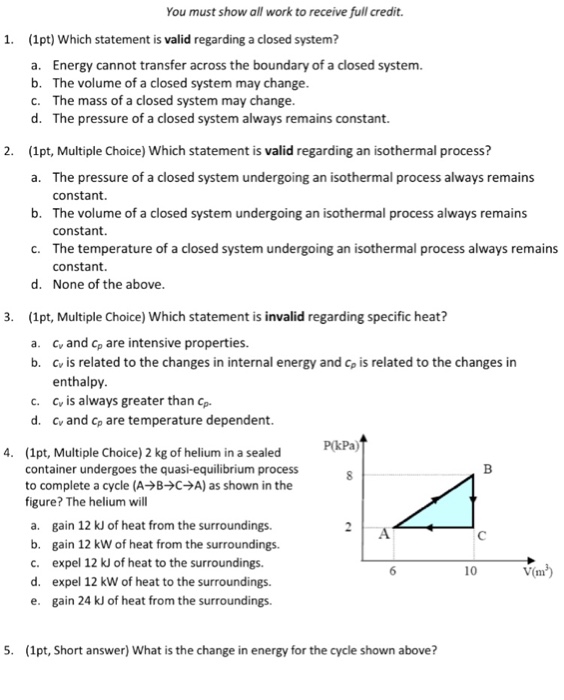
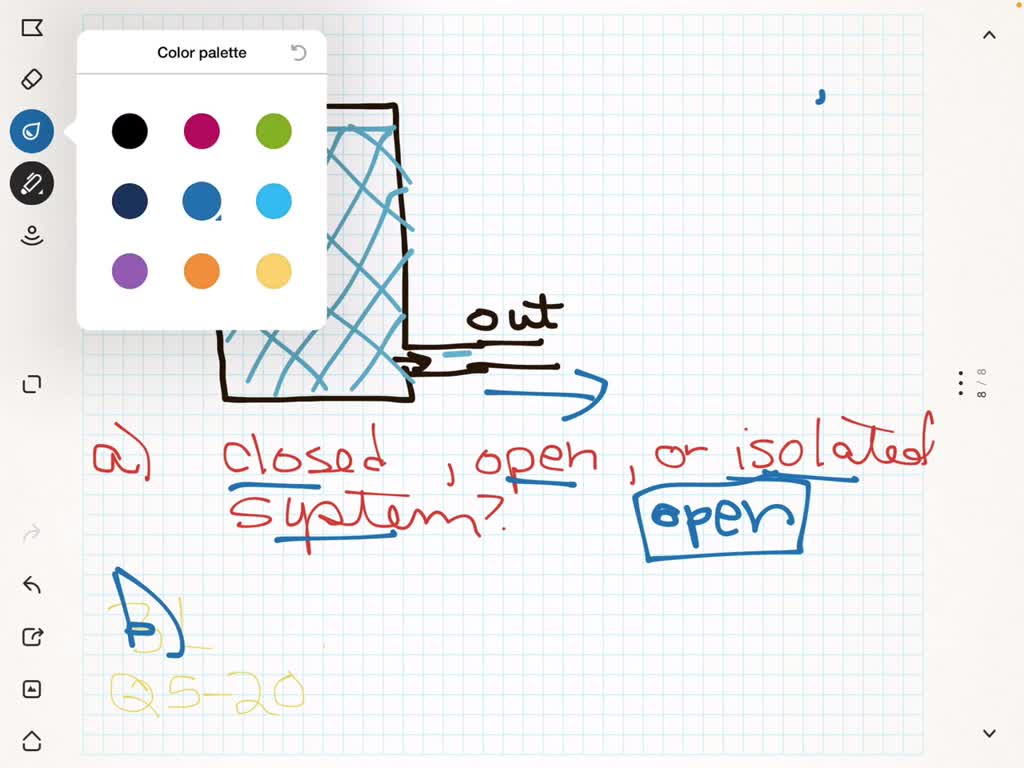
Heat rejection is the only way that the entropy of a fixed mass can be decreased. -----b The volume of a closed system can change. To sum it up- we can write -. You introduce a control volume so that you can focus on what happens inside it but you must take account of anything that passes through the control volume. It can do anything you want it to do. But in the absence of charge by the very definition of work PdV then. The change in concentration is used to calculate system volume. Now consider mass flow into or out of the control volume through a differ-ential area dAon the control surface of a fixed control volume. An isochoric process also called a constant-volume process an isovolumetric process or an isometric process is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant.
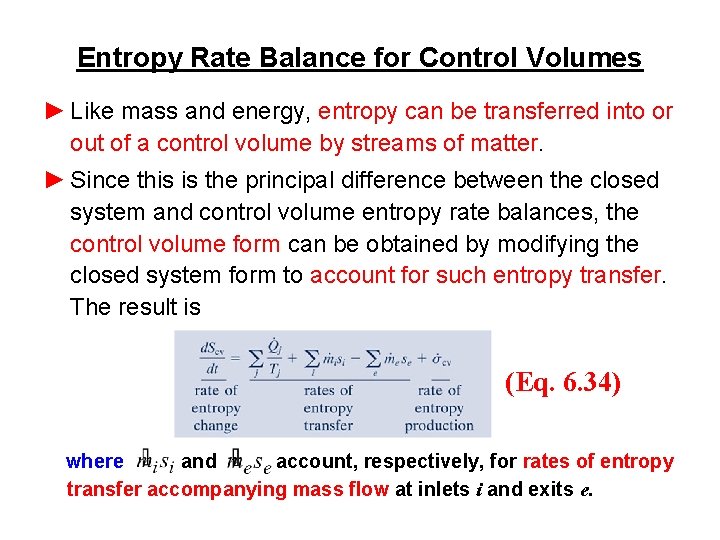
-----b The volume of a closed system can change. Entropy can be transferred to or from a system in two forms. This rela-tion is valid whether the control volume is fixed moving or deforming. Without moreinformation this is about the best answer we can provide. The entropy of a closed system can decrease. Thus the entropy transfer for an adiabatic closed system is zero. To sum it up- we can write -.


























Post a Comment for "Can The Volume Of A Closed System Change"